When Professor Sir Nick White arrived in Thailand from Oxford University 40 years ago to join a small medical research whole, his duties included treating malaria and milking deadly snakes. today, from his base at Bangkok ’ s Mahidol University, he leads an international team conducting a worldwide COVID-19 drug trial .
During Mr. White ’ s four decades in Thailand, the Mahidol Oxford Tropical Medicine Research Unit ( MORU ) —a collaboration between Oxford and Mahidol Universities and the London-based Wellcome Trust—has grown from three researchers to 600. And its activities have spread far beyond Thailand to countries across Asia and Africa. now, having pioneered treatments for baneful tropical diseases, MORU is enrolling 40,000 frontline healthcare workers in what is the world ’ s only large-scale clinical trial to test whether the drugs hydroxychloroquine and chloroquine can prevent COVID-19 .
“ We are doing this from Thailand because we have the experience here to conduct large, multinational trials, ” says Mr. White, who holds professorships at both Oxford and Mahidol, and who was knighted in 2017 by the U.K. ’ s Queen Elizabeth for his service to ball-shaped health. “ Thailand punches above its burden in clinical checkup inquiry. It has very good academic standards and has created a milieu that prizes invention, creativity and cognition. ”
Thailand has hanker been internationally recognized as a destination for alleged medical tourists, lured by Bangkok ’ s first hospitals. Less well-known, however, is its fundamental healthcare ecosystem that is helping to transform Southeast Asia ’ mho second largest economy into a much broader, more divers medical hub. nowadays, it attracts investors and researchers in biotechnology—which includes vaccines, genomics and biopharmaceuticals—as well as high-tech medical devices .
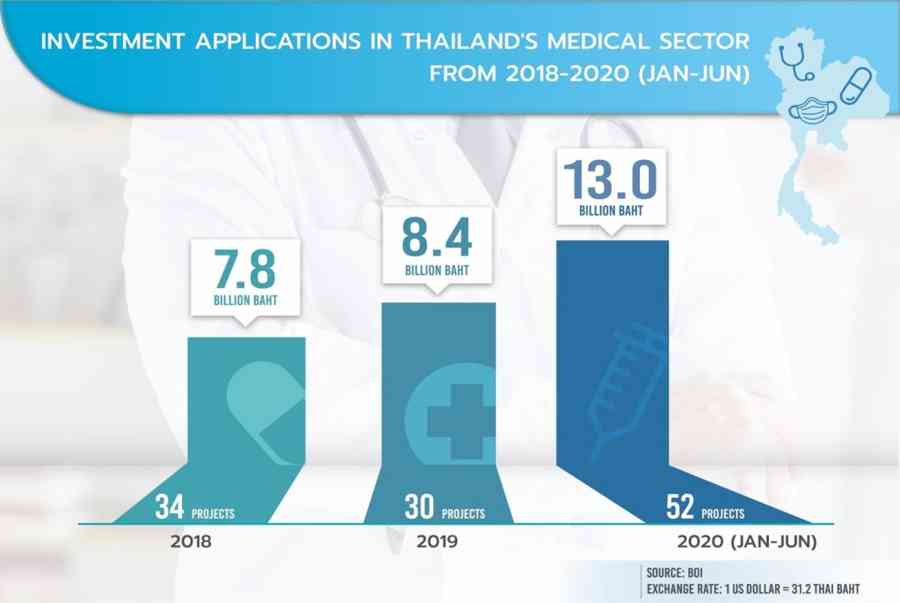
While the COVID-19 pandemic has crippled industries in other parts of the global, it has served to raise investor confidence in Thailand ’ randomness healthcare sector. In the beginning one-half of 2020, 52 medical-related businesses with projects worth more than $ 400 million sought blessing from Thailand ’ s Board of Investment ( BOI ) —an increase of 170 % in undertaking numbers and 123 % in value over the same period last year .

In one recent exercise, Genexine Inc.—South Korea ’ randomness largest biotechnology company with a market value of $ 2.5 billion—teamed up with a Thai collaborator to establish KinGen Biotech, a manufacturer of oncology drugs and DNA vaccines for cervical cancer .
KinGen is targeting annual sales of $ 250 million by 2026. “ The BOI is very supportive of our project, ” says Genexine ’ s CFO Kim Kyu Don. “ Our sales finish is ambitious but besides very feasible. ” KinGen aims to use Thailand as a research and development hub, ampere well as production base for Southeast Asia, the Middle East, Africa, Latin America and Russia. “ Thailand ’ s aesculapian sector has big potential, ” Mr. Kim says. “ We want to transfer engineering to help it develop further. ”
Another enterprise that has just received BOI back is Apsalagen Co. Ltd, a joint venture between Thai-owned Siam Bioscience and Germany ’ s Haase Investment, the chief stockholder in Berlin-based Biotechrabbit GmbH. The partnership produces biological reagents and master mixes used in the production of rapid tests ( RT-PCR ) for the detection of COVID-19 .
Thailand ’ s achiever at attracting medical sector investment stems in big separate from two key factors. The first is the raft of incentives offered by its BOI to qualifying Thai and foreign-owned companies, including tax breaks for up to 13 years and smart visa that enable expatriate researchers and early keystone employees and their families to stay in the nation for up to four years. The second is Thailand ’ s path record both in containing the spread of COVID-19 and in developing vaccines .
Since the pandemic began, Thailand has won international praise for the gloomy infection rate among its population of closely 70 million. Over the same period, Thailand has besides become one of the first middle-income nations to begin clinical trials on a home-developed COVID-19 vaccine .
separate from the ongoing Oxford-Mahidol ball-shaped trial on hydroxychloroquine and chloroquine, Thai universities and biotechnology companies are working on at least three early vaccine candidates.
In the most advanced of these, Chulalongkorn University ’ s Chula Vaccine Research Center is about to begin human trials on a vaccine that has already been tested successfully on mice and monkeys .
Since its establish 15 years ago, Chula VRC has worked on vaccines to combat health scourges such as HIV and dengue fever, forging collaborations with several U.S. government and academic institutions including the National Institute of Health and the University of Pennsylvania .
now, its COVID-19 vaccine, based on a engineering known as messenger rna, is attracting international media attention. And if the approaching human trials prove successful, the team led by Dr. Kiat Ruxrungtham hopes to work with manufacturers in Thailand, North America and Germany to produce 30 million doses—enough to supply Thailand and six other asian countries .
Dr. Kiat says his team is besides collaborating with a second Chulalongkorn study in which Dr. Thiravat Hemachudha of the Thai Red Cross Emerging Infectious Disease Health Science Center in collaboration with Baiya Phytopharm, a spinoff from the university ’ s faculty of pharmaceutical sciences, has developed a potential vaccine that uses a unlike technology derived from plant protein. “ It ’ randomness big to have more than one vaccine campaigner in Thailand among 180 candidates globally, ” Dr. Kiat says .
 simultaneously, the Bangkok-based, French-Thai joint guess BioNet-Asia is both working on its own COVID gene-based vaccine, which is about to begin human trials in Australia, and collaborating with Thai and U.S. universities to manufacture millions of doses of another genetic vaccine .
simultaneously, the Bangkok-based, French-Thai joint guess BioNet-Asia is both working on its own COVID gene-based vaccine, which is about to begin human trials in Australia, and collaborating with Thai and U.S. universities to manufacture millions of doses of another genetic vaccine .
Founded in 2001, BioNet-Asia opened a research and development center in 2009, and has since then been developing recombinant vaccines against infectious diseases ranging from whooping cough, dengue fever and Zika—the latter in partnership with the esteemed Pasteur Institute in Paris .
This year, BioNet set aside all early work to focus its 250-strong work force on developing and producing a COVID-19 vaccine using recombinant DNA engineering .
BioNet CEO Pham Hong Thai says that while many COVID-19 researchers in other countries have to team up with drug manufacturers to produce the end intersection, his company is able to not alone develop but besides industry vaccines. “ Within one site, we had researchers and production teams working hand in hand to produce promptly the fabricate batches, which will be promote evaluated in clinical trials in different countries as part of a ball-shaped collaboration, ” he says .
back at the Oxford-Mahidol University MORU collaboration, Mr. White ’ mho Bangkok-based team is working on the global cogitation it hopes will last determine whether hydroxychloroquine and chloroquine—used for 60 years to treat malaria and rheumatological conditions—can prevent COVID-19.
Read more: Ex on the Beach (British series 6)
Mr. White believes he is in the right place to do it. “ In terms of a hub for an international collaboration, Thailand is identical good, ” he says. “ COVID-19 has shown that the earth is a very small space. Countries can not just look inwards, and Thailand has the vision to understand the importance of global health angstrom well as national health. ”
Learn more about Thailand Board of Investment here .
Wall Street Journal Custom Content is a unit of The Wall Street Journal Advertising Department. The Wall Street Journal news arrangement was not involved in the creation of this contented .